Heterogenous catalysis is the chemical reaction where the phase of catalysts differs from that of the reactants or products. Catalysts are used to increase the rate, effectivity and selectivity of reactions, thus enabling a large-scale product formation. Examples of such reactions are oxidation of CO on the surface of platinum, dissociation of molecular oxygen and hydrogen in the hydrogen fuel cell (electrocatalysis) or Haber-Bosch synthesis of ammonia.
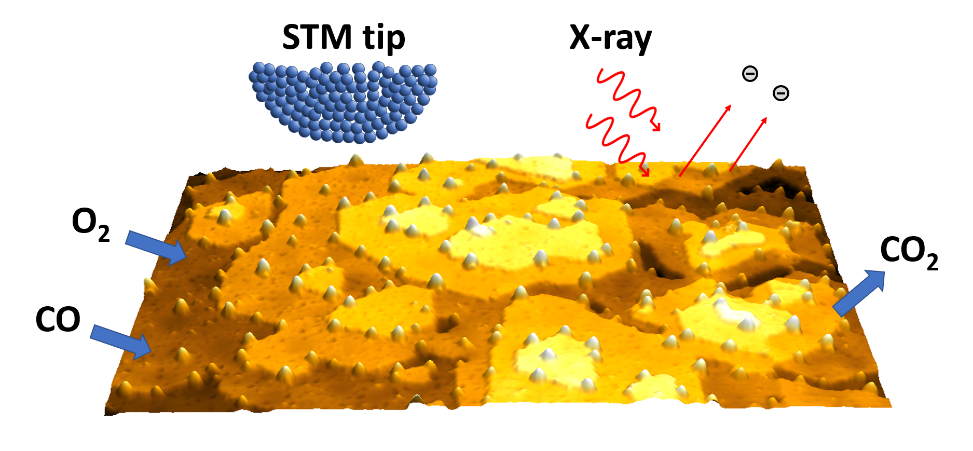
To further increase the speed of reactions, most of the industrial heterogeneous catalysis is executed in high reactant pressure and temperature. This strongly contrasts with a huge body of experimental studies focused on catalyst surfaces in ultra-high vacuum conditions and relatively low temperature, i.e. in conditions that enable use of sensitive spectroscopic and microscopic techniques. Only lately, it has been shown that even simplest metallic catalyst surfaces significantly reconstruct under high pressure and temperature of reactants. This has led to emergence of a new branch of experimental surface science techniques, which are able to observe surfaces under so-called operando conditions. The techniques are often referred to as near-ambient pressure (NAP) techniques.
This dissertation thesis will focus on a combination of multiple NAP techniques: NAP scanning tunneling microscopy (NAP-STM), NAP scanning force microscopy (NAP-AFM), NAP X-ray photoelectron spectroscopy (NAP-XPS) and NAP ultraviolet photoelectron spectroscopy (NAP-UPS). All the methods are combined into one ultra-high vacuum apparatus and thus can be used in-situ. This unique combination of methods will be used to characterize catalytic surfaces and their topographic and chemical changes directly during the ongoing surface reactions.

| peter.matvija@mff.cuni.cz | |
| +420-95155-2749 +420-95155-2671 |

Lectures, interesting facts and information in the field of nanomaterials and hydrogen technology.
© 2021 Matematicko-fyzikální fakulta Univerzity Karlovy.
Všechna práva vyhrazena. | Cookies