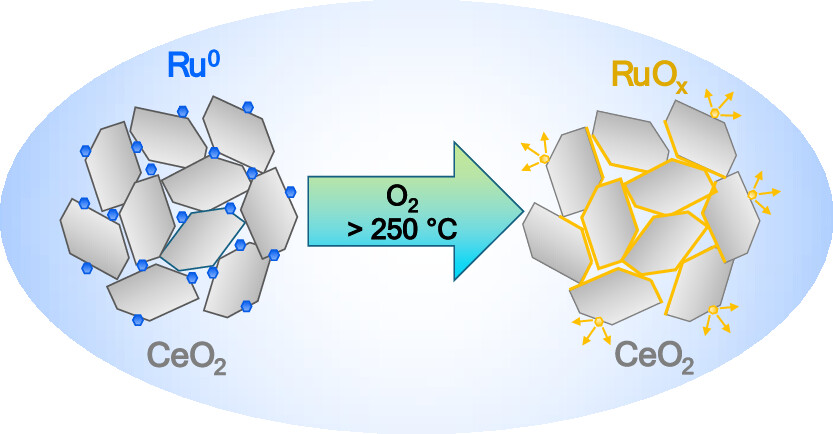
This work investigates the surface chemistry of the Ru/CeO2 catalyst under varying pretreatment conditions and during the oxidation of propane, focusing on both dry and humid environments. Our results show that the Ru/CeO2 catalyst calcined in O2 at 500 °C initiates propane oxidation at 200 °C, achieves high conversion rates above 400 °C, and demonstrates almost no change in activity in the presence of water vapor across the entire studied temperature range of 200–500 °C. Prereduction of the oxidized Ru/CeO2 catalyst in H2 significantly enhances its activity, though this enhancement diminishes at higher temperatures. Adding water to the reaction mixture boosts the low-temperature activity of the prereduced catalyst but decreases it at 300–400 °C. Several ex-situ analytical techniques in combination with the in-situ NAP-XPS analysis reveal that while exposed to oxygen, Ru nanoparticles on the ceria surface oxidize to form RuO2 below 200 °C and volatile RuOx (x > 2) at higher temperatures. The presence of water vapor in the reaction mixture leads to the transformation of RuO2 into ruthenium hydroxide at 200 °C, which, in turn, facilitates propane oxidation. At higher temperatures, the water does not have much influence on the oxidation state of Ru but slightly inhibits its evaporation from the surface. It is also demonstrated that Ru in the Ru/CeO2 catalyst exists predominantly in the Run+ (n > 4) oxidation states at typical VOC oxidation temperatures rather than the expected Ru4+ state.
More information can be found here: https://doi.org/10.1021/acs.jpcc.4c08033.
© 2021 Matematicko-fyzikální fakulta Univerzity Karlovy.
Všechna práva vyhrazena. | Cookies