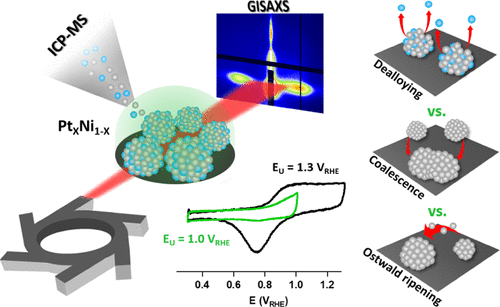
Platinum-based bimetallic alloys have been largely investigated during the last few years as a valid alternative to bare Pt cathode catalysts for proton-exchange membrane fuel cells (PEMFCs) to improve their cost-efficiency. Nonetheless, Pt bimetallic alloys are characterized by a reduced stability, which is poorly understood at a fundamental level. It is thus essential to describe the entire chain of interconnected degradation mechanisms to formulate a comprehensive model of catalyst degradation that will help interpret bimetallic alloy behavior in real complex fuel cell systems. By combining in situ inductively coupled plasma mass spectroscopy, in situ grazing-incidence small-angle X-ray scattering, and ex situ scanning electron microscopy, we have studied the morphological evolution of PtXNi100–X model catalysts with different Ni contents (ranging from 0 to 75%) undergoing potentiodynamic cycling to two different upper potentials mimicking the different operational conditions of a PEMFC: 1.0 and 1.3 VRHE. Data analysis allowed us to develop a methodology to distinguish the influence of Ni dissolution, particle coalescence, and Ostwald ripening on particle size distribution and interparticle distance and to realize time-dependent interplay maps to highlight the timeframe in which the aforementioned phenomena are prevailing or coexisting. Results show that Ni dissolution is the only phenomenon inducing morphological evolution when the lower upper potential is chosen. On the contrary, at 1.3 VRHE, Ni dissolution is rapidly overcome by particle coalescence at first and by Ostwald ripening in the later stages of the investigated time range. The onset of every phenomenon was found to occur earlier in time for larger values of Ni concentrations.
© 2021 Matematicko-fyzikální fakulta Univerzity Karlovy.
Všechna práva vyhrazena. | Cookies